Chapter 4 – Chemical Kinetics Questions and Answers: NCERT Chemical Kinetics for Class 12 Chemistry
Class 12 Chemistry chapter 4 - Chemical Kinetics - Questions and Answers of NCERT Book Solutions.
4.1.For the reaction R—>P, the concentration of reactant changes from 0.03 M to 0.02 M in 25 minutes. Calculate the average rate of reaction using units of time both in minutes and seconds.
Ans.
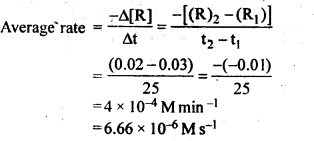
4.2.In a reaction, 2A —-> Products, the concentration of A decreases from 0.5 mol L-1 to 0.4 molL-1 in 10 minutes. Calculate the rate during this interval?
Ans.
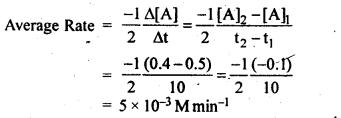
4.3. For a reaction, A + B → Products, the rate law is given by : r = k [A]1/2[B]2. What is the order of reaction?
Ans. Rate law(r) = k [A]1/2[B]2
order of reaction = 12+2=212or2.5
4.4.The conversion of molecules X to Y follows second order kinetics. If concentration of X is increased to three times how will it affect the rate of formation of Y ?
Ans.The reaction is : X—>Y
According to rate law,
rate = k[X]2
If [X] is increased to 3 times, then the new rate is
rate’ = k[3X]2
rate’ = 9 k [X]2 = 9 rate
Thus, rate of reaction becomes 9 times and hence rate of formation of Y increases 9-times.
4.5. A first order reaction has a rate constant 1.15 x 10-3 s-1. How long will 5 g of this reactant take to reduce to 3 g?
Ans.
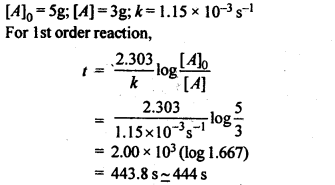
4.6.Time required to decompose SO2Cl2 to half of its initial amount is 60 minutes. If the decomposition is a first order reaction, calculate the rate constant of the reaction.
Ans. For 1st order reaction,
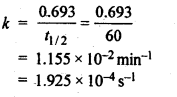
4.7. What will be the effect of temperature on rate constant?
Ans. In general, the rate constant for a reaction nearly becomes double with about 10° rise in temperature because of the fact that the effective collisions become almost double. The exact dependence of the reaction rate on temperature is given by Arrhenius equation; k=Ae−Ea/Rt.Where A is the Arrhenius factor or the frequency factor. It is also called pre exponential factor. It is a constant specific to a particular reaction. R is gas constant and Ea is activation energy measured in joules/mole (J mol-1).
4.8.The rate of the chemical reaction doubles for and increase of 10 K in absolute temperature from 298 K. Calculate Ea.
Ans.

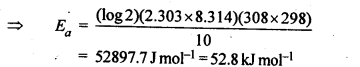
4.9.The activation energy for the reaction, 2 HI(g) —-> H2+I2 (g) is 209.5 k J mol-1 at 581 K.Calculate the fraction of molecules of reactants having energy equal to or greater than activation energy?
Ans.Fraction of molecules having energy equal to or greater than activation energy is given by:
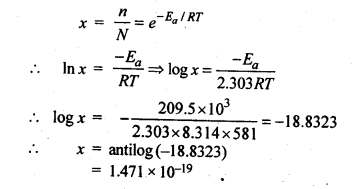
Last Updated on: Feb 23, 2024
