Question: Identify the types of reaction mentioned above in (i) and (ii). Give one example for each type in the form of a balanced chemical equation.

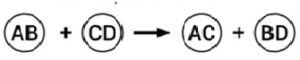
The correct answer is :
i) Displacement – ½ M
● Fe(s) + CuSO4(aq) → FeSO4(aq) + Cu(s) (1 mark)
● Zn(s) + CuSO4(aq)→ ZnSO4(aq) + Cu(s)
● Pb(s) + CuCl2(aq) → PbCl2(aq) + Cu(s)
(Any one of the reaction or other displacement reaction.)
ii) Double displacement (½ mark)
Na2SO4 (aq) + BaCl2(aq) → BaSO4 (s) + 2NaCl(aq) (1 mark)
(Any one of the reaction or other double displacement reaction.)


